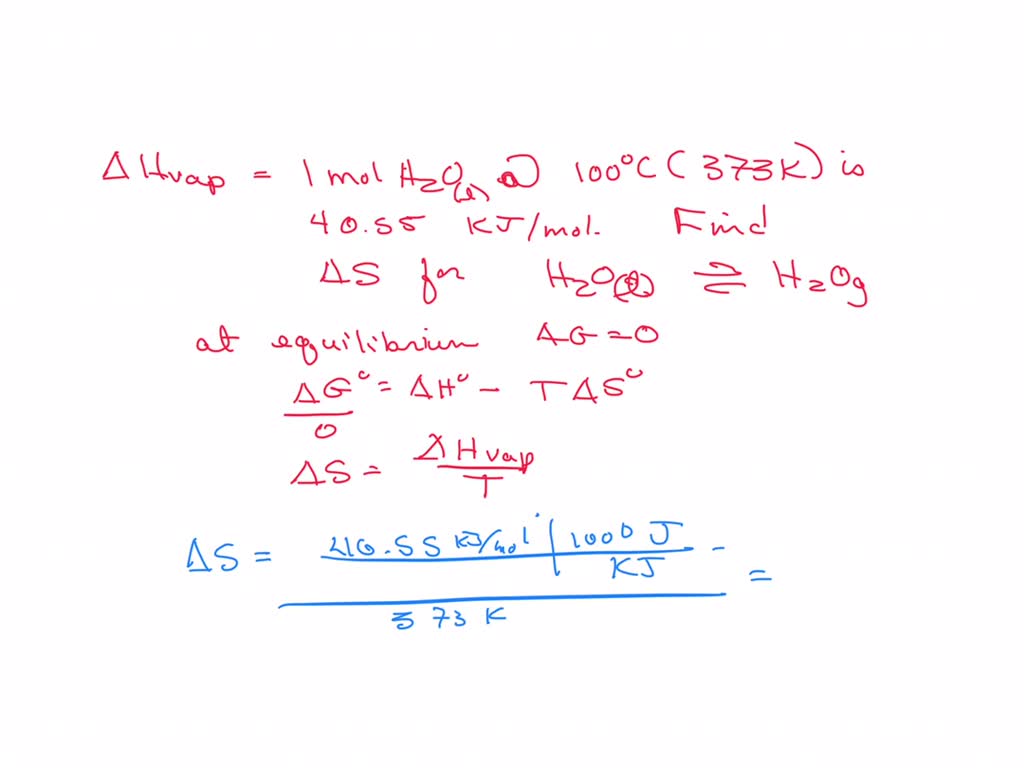
SOLVED: The heat of vaporization for 1.0 mole of water at 100°C and 1.0 atm is 40.55 kJ/mol. Calculate ΔS for the process H2O(l) â†' H2O(g) at 100°C.

The enthalpy of vaporization of a substance is 840J/mole and its boiling point is -173C its entropy of vaporization is - Chemistry - Thermodynamics - 13308591 | Meritnation.com
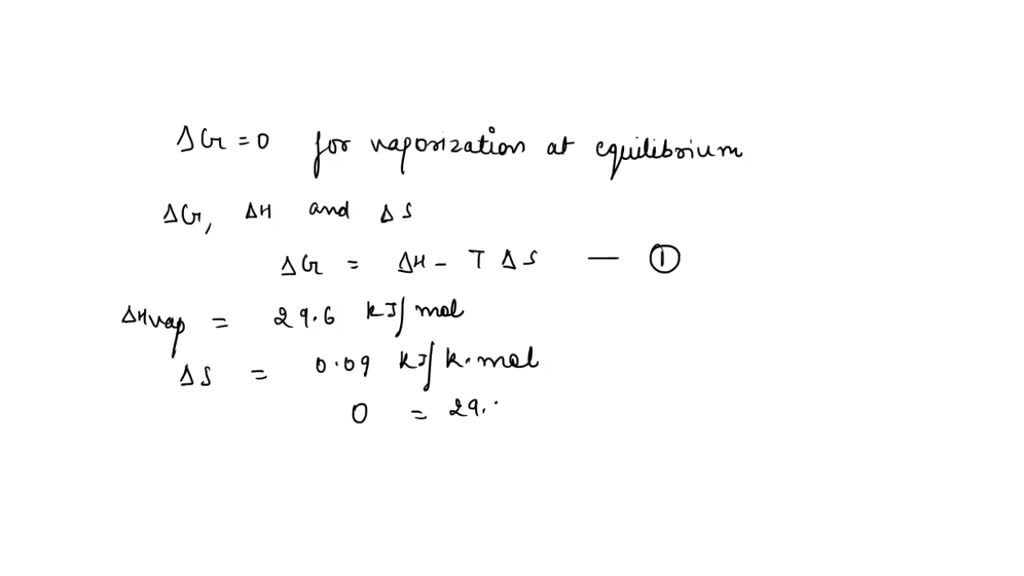
SOLVED: The entropy increase in the vaporization of 1.00 mole of bromine is 0.09 kJ/K. If the heat of vaporization is 29.6 kJ/mol, what is the normal boiling point of bromine in

The Clinic Ave Pampanga - Mole Removal before and right after laser vaporization procedure. Call or text us at 09173038027 for appt or PM us here on FB. :) #TheClinicAve #MedicalAesthetics #Pampanga #
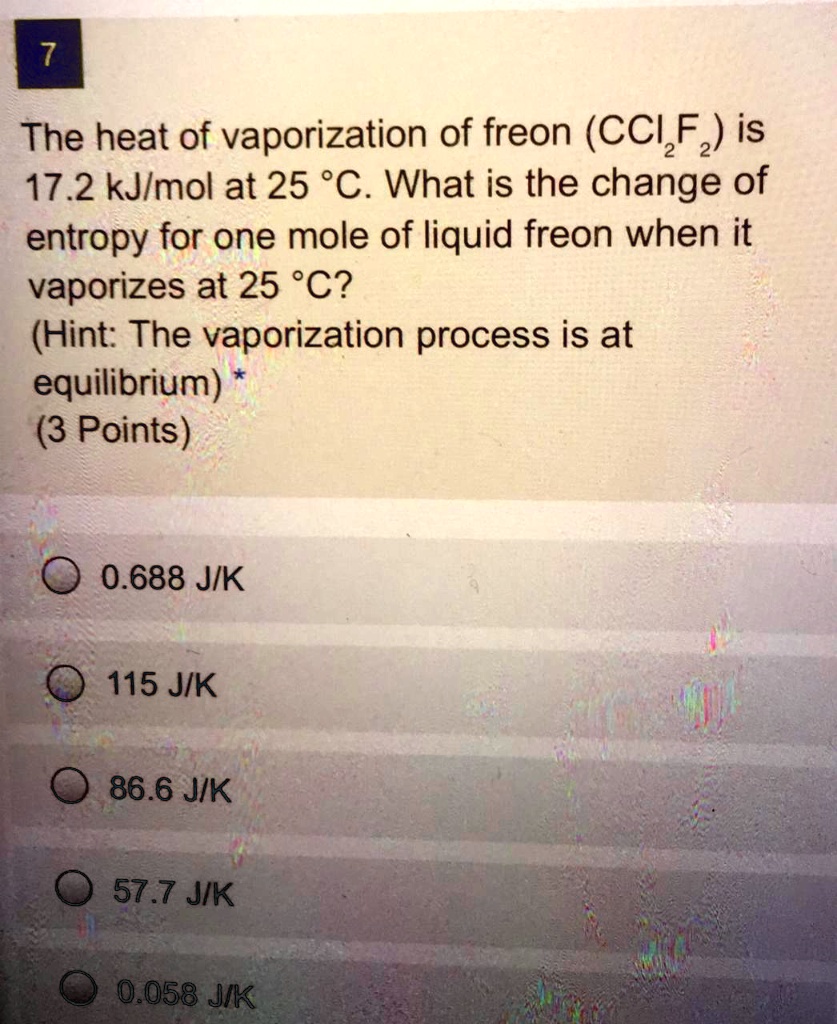
SOLVED: The heat of vaporization of freon (CCl2F2) is 17.2 kJ/mol at 25 °C. What is the change of entropy for one mole of liquid freon when it vaporizes at 25 °C? (
The entropy of vaporization of benzene is 85 JK^ 1mol^ 1. When 117 g benzene vaporizes at its normal boiling point then the entropy change of surrounding is : (1) 85 JK^ 1 (2) 85 × 1.5 JK^ 1 (3 ) 85 × 1.5 JK^ 1 (4) None of these .

What does the latent heat of vaporization represent? A. The amount of energy required to turn a mole of a - brainly.com